br electronic configuration|Electron Configuration Chart of All Elements (Full Chart) : Manila How can I figure out the electron configuration of Br-? DAVAO CITY – The local government has opened hotline numbers where Dabawenyos can call or send messages on vaccine-related questions and concerns. Dr. Josephine Villafuerte, head of the .
br electronic configuration,Complete Electron Configuration for Bromine (Br, Br- ion)How can I figure out the electron configuration of Br-?How can I figure out the electron configuration of Br-?
Complete Electron Configuration for Bromine (Br, Br- ion)
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure \(\PageIndex{2}\)): The number of the .Electron Configuration Chart of All Elements (Full Chart) In the spin up configuration, the hybridized d t2g –Nb and the p-Cl/Br states reside in the Fermi level, resulting in metallic behavior for the studied variant perovskites.br electronic configuration In this video we will write the electron configuration for Br-, the Bromide ion. We’ll also look at why Bromine forms a 1- ion and how the electron configura.br electronic configuration Electron Configuration Chart of All Elements (Full Chart) In this video we will write the electron configuration for Br-, the Bromide ion. We’ll also look at why Bromine forms a 1- ion and how the electron configura. March 23, 2023 Jay. Electron configuration chart of all .
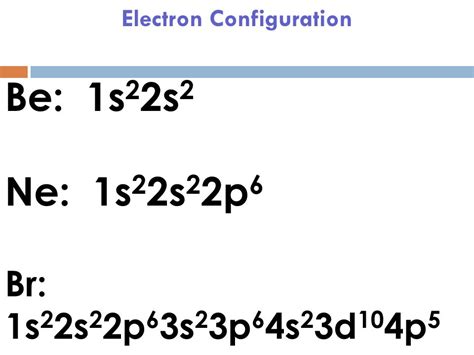
The properties of chlorine are intermediate between those of iodine and chlorine. Bromine Orbital Diagram. In this article today we are going to tell you about the electron configuration of Bromine, its orbital .The electronic configuration for $\ce{Br-}$ is: $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ Because it have one more electron than bromine, which ends its .Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main .Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Define paramagnetism and diamagnetism. Justify the .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to . The electron configuration of Bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[Ar] 4s^2 3d^10 4p^5#. Explanation: Use a chart such as the one below to fill the .Reduced electronic configuration Br: [Ar] 3d 10 4s 2 4p 5. Below is the electronic diagram of the Bromine atom Distribution of electrons over energy levels in the Br atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st level (N): 7. Valence electrons of Bromine.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.The chemical symbol for Bromine is Br. Electron Configuration and Oxidation States of Bromine. Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of .Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . CH 3 Br (boiling point 3.5 o C), this has been widely employed to kill pests in the soil, in storage facilities .
Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to .

The condensed electronic configuration of Br will be Ar 18 4s 2 3d 10 4p 5. Conclusion . A reddish-brown liquid, bromine has a strong odor that irritates the skin, eyes, and respiratory system. It belongs to group 17 of the periodic table’s halogen family. Even a brief period of exposure to concentrated bromine has the potential to be .Click here:point_up_2:to get an answer to your question :writing_hand:configuration of displaystyle bris displaystyle left ar right 3d104s24p6 the electronic configuration ofdisplaystyle br2
When writing an electron configuration, you have to write serially. Ruthenium ion(Ru 3+) electron configuration. The ground state electron configuration of ruthenium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 .Write out the full electron configuration for each of the following atoms and for the monatomic ion found in binary ionic compounds containing the element: Al; Br; Sr; Li; As; S; Answer a. Al: 1s 2 2s 2 2p 6 3s 2 3p 1. Al 3+: 1s 2 2s 2 2p 6. Answer b. Br: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Br-: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, .
Bromine - 35 Br Your user agent does not support the HTML5 Audio element. 🔊 🇬🇧 Bromine . The ground state electron configuration of ground state gaseous neutral bromine is [Ar].3d 10.4s 2.4p 5 and the term symbol is 2 P 3/2. Schematic electronic configuration of bromine.
What is the electronic configuration of Bromine & Iodine? In study material last topic last video it is given 2,8,18,7 & 2,8,18,18,7 which I think so is wrong ? View Solution. Q4. Write out the electron configuration for neutral bromine. .A Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already . Given: Bromine (Br) Asked for: S, the shielding constant, for a 3d electron. Strategy: Determine the electron configuration of bromine, then write it in the appropriate form. Use the appropriate Slater Rule to calculate the shielding constant for the electron. Solution A Br: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Br: (1s 2)(2s 2,2p 6)(3s 2 . What is the electron configuration for bromine? Chemistry Electron Configuration Electron Configuration. 1 Answer Zach Dec 24, 2015 [Ar] #4s^(2)3d^(10)4p^5# Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full .
br electronic configuration|Electron Configuration Chart of All Elements (Full Chart)
PH0 · How can I figure out the electron configuration of Br
PH1 · First
PH2 · Electronic configuration of bromine ?
PH3 · Electron Configuration Chart of All Elements (Full Chart)
PH4 · Complete Electron Configuration for Bromine (Br, Br
PH5 · Bromine Electron Configuration (Br) with Orbital Diagram
PH6 · Bromine
PH7 · Br
PH8 · 7.4: Electron Configurations of Ions
PH9 · 3.3: Electronic Structure of Atoms (Electron Configurations)
PH10 · 2.4 Electron Configurations